The national referral center
for neuro-inflammatory diseases
The national referral center
for neuro-inflammatory diseases
THE NATIONAL CENTER
Mircem is the national referral center for neuro-inflammatory diseases register all data of various inflammatory neurological diseases.
Its purpose is to inform the public and professionals on the knowledge of these diseases and to establish and propose available therapies.
It coordinates 2 constituent centers and 14 competence centers.
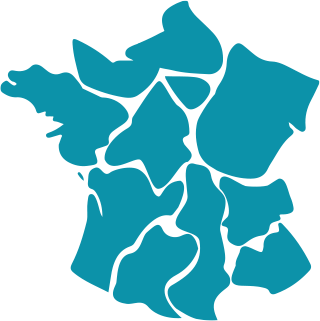
• Competence center of Lille
Dr Jean-Christophe CUVELLIER
Pediatric Neurology Department
CHU Lille
Hôpital Jeanne de Flandre<
2 avenue Oscar Lambret
59037 Lille Cedex
• Competence center of Amiens-Picardie
Dr Anne-Gaëlle LE MOING
Pediatric Neurology Department
CHU d’Amiens-Picardie
Hôpital Nord
Place Victor Pauchet
80054 Amiens Cedex 1
• Competence center of Besançon
Dr Caroline PARIS
Pediatric Medicine Department – CHRU de Besançon
Hôpital Jean Minjoz
3 Boulevard Alexandre-Fleming
25030 Besançon Cedex<
• Competence center of Tours
Pr Pierre THOMAS-CASTELNAU
Neuropediatrics and Disabilities Department – CHRU de Tours
Hôpital Clocheville
49 Boulevard Béranger
37044 Tours Cedex 9
• Constituent center of Lyon
Pr Romain MARIGNIER
Neurology Department – Multiple Sclerosis, Myelin Disease and Neuroinflammation
Hospices Civils de Lyon
Hôpital Pierre Wertheimer
59 Boulevard Pinel
69677 Bron Cedex
• Competence center of Bordeaux
Dr Fréderic VILLEGA
Medical Pediatrics Department – CHU de Bordeaux
Place Amélie Raba-Léon
33076 Bordeaux Cedex
• Competence center of Marseille
Dr Anne LEPINE
Pediatric Neurology Department – Pédiatrie spécialisée & médecine infantile
CHU de Marseille
Hôpital de la Timone
13005 Marseille
• Competence center of Brest
Dr Sylviane PEUDENIER
Pediatrics Department – CHRU de Brest
Hôpital Morvan
2 Avenue Foch
29609 Brest Cedex
• Competence center of Nancy
Dr Hélène VINCENT
Pediatrics Department – children and teenagers
CHU de Nancy
Rue du Morvan
54511 Vandœuvre-Lès-Nancy Cedex
• Competence center of Strasbourg
Dr Anne DE SAINT-MARTIN
Neuropediatrics Department – Pédiatrie médico-chirurgicale
CHU de Strasbourg
Hôpital de Hautepierre
1 Avenue Molière
67200 Strasbourg
• Competence center of Montpellier
Dr Pierre MEYER
Specialized Pediatric Department – CHU de Montpellier
Hôpital Gui de Chauliac
80 avenue Augustin Fliche
34295 Montpellier Cedex 5
• Competence center of Toulouse
Dr Emmanuel CHEURET
Pediatrics Department – Neurologie et infectiologie
CHU de Toulouse
Hôpital des Enfants
330 Avenue de Grande Bretagne
31059 Toulouse Cedex 9
• Constituent center of The Pitié-Salpêtrière
Dr Anne-Caroline PAPEIX
Multiple Sclerosis and Inflammatory Disease Unit – AP-HP. Sorbonne Université
Hôpital de la Pitié-Salpêtrière
47-83 boulevard de l’Hôpital
75013 Paris
• Competence center of Armand-Trousseau
Dr Diane DOUMMAR
Neuropediatrics Department – AP-HP. Sorbonne Université
Hôpital d’Enfants Armand-Trousseau
26 avenue du Docteur Arnold Netter
75012 Paris Cedex 12
• Competence center of Necker
Pr Isabelle DESGUERRE
Pediatric Neurology Department – AP-HP. Centre – Université de Paris
Hôpital Necker-Enfants Malades
149 rue de Sèvres
75743 Paris
• Competence center of Robert Debré
Dr Florence RENALDO
Pediatric neurology and metabolic diseases department – AP-HP. Nord – Université de Paris
Hôpital Robert Debré
48 boulevard Sérurier
75019 Paris
THE MIRCEM TEAM
PUBLISHED WORK
Our team, made up of experts in the management of rare inflammatory diseases of the brain and marrow, develops research projects and has published
numerous scientific articles.
MIRCEM paediatrics Multidisciplinary Consultation Meetings (RCP)
The RCP are a meeting place between different health professionals who consult and discuss collegially around the most complex patient files in order to propose an adequate diagnostic and treatment plan.
RCP are organized monthly via the Rofim platform.
The next meetings will take place:
• Wednesday September 22, 2021 at 2 p.m.
• Wednesday 20 October 2021 at 2 p.m.
• Wednesday November 24, 2021 at 2 p.m.
• Wednesday December 15, 2021 at 2 p.m.
• Wednesday January 19, 2022 at 2 p.m.
• Wednesday February 16, 2022 at 2 p.m.
• Wednesday March 16, 2022 at 2 p.m.
• Wednesday April 13, 2022 at 2 p.m.
• Wednesday May 18, 2022 at 2 p.m.
• Wednesday June 15, 2022 at 2 p.m.
For professionals who wish to participate, please contact us.
The RCP are a meeting place between different health professionals who consult and discuss collegially around the most complex patient files in order to…
Annals of Neurology – January 2021
The main objective was to compare clinical features, disease course, and myelin oligodendrocyte glycoprotein (MOG) antibody (Ab) dynamics between children and adults with MOG-Ab-associated disease (MOGAD).
This retrospective multicentric, national study included 98 children and 268 adults with MOGAD.
Isolated optic neuritis was the most frequent clinical presentation in both children (40.8%) and adults (55.9%), and acute disseminated encephalomyelitis syndrome was more frequent in children (36.7% vs 5.6%). Compared to adults, children displayed better recovery.
At 2 years, 64.2% of nonrelapsing children became MOG-Ab negative compared to 14.1% of relapsing children, with no differences observed in adults.
MOGAD patients differ in the clinical presentation at onset, showing an age-related shift in the clinical features across age groups. Compared to children, adults have a higher risk of relapse and worse functional recovery. Finally, children with monophasic disease become MOG-Ab negative earlier than relapsing children, but this is not true in adults.
Cobo-Calvo A, Ruiz A, Rollot F, et al. Clinical Features and Risk of Relapse in Children and Adults with Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease. Ann Neurol. 2021 Jan;89(1):30-41.
The main objective was to compare clinical features, disease course, and myelin oligodendrocyte glycoprotein (MOG) antibody (Ab) dynamics between children and…
JAMA Neurology - September 2020
Risk factors associated with the severity of coronavirus disease 2019 (COVID-19) in patients with multiple sclerosis (MS) are unknown. Describe the clinical characteristics and outcomes in patients with MS and COVID-19 and identify factors associated with COVID-19 severity are important to provide the rationale for an individual strategy regarding clinical management of this patients during the COVID-19 pandemic.
It is in this vision that the Covisep register takes place. The study is a multicenter, retrospective, observational cohort study, included 347 patients with MS presenting with a confirmed or highly suspected diagnosis of COVID-19 between March 1, 2020, and May 21, 2020.
The main outcome was COVID-19 severity assessed on a 7-point ordinal scale (ranging from 1 [not hospitalized with no limitations on activities] to 7 [death]) with a cutoff at 3 (hospitalized and not requiring supplemental oxygen).
Demographics, neurological history, Expanded Disability Severity Scale score (EDSS; ranging from 0 to 10, with cutoffs at 3 and 6), comorbidities, COVID-19 characteristics, and outcomes were collected.
21.0% of patients had a COVID-19 severity score of 3 or more, and 3.5% of patients died of COVID-19. 81.8% of patients were receiving DMT.
There was a higher proportion of patients with a COVID-19 severity score of 3 or more among patients with no DMT relative to patients receiving DMTs (46.0% vs 15.5%). Multivariate logistic regression models determined that age, EDSS, and obesity were independent risk factors for a COVID-19 severity score of 3 or more. The EDSS was associated with the highest variability of COVID-19 severe outcome, followed by age and obesity. There was no association found between DMTs exposure and COVID-19 severity.
Louapre C, Collongues N, Stankoff B, et al. Clinical Characteristics and Outcomes in Patients With Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020 Sep 1;77(9):1079-1088.
Risk factors associated with the severity of coronavirus disease 2019 (COVID-19) in patients with multiple sclerosis (MS) are unknown. Describe the…
Neurol Neuroimmunol Neuroinflamm - July 2020
Retrospective, multicenter, and multinational study of patients with AQP4-Ab NMOSD aged <18 years at disease onset from a center in Brazil and 13 European centers.
A total of 67 children were identified. 57.8%) were found to have permanent disability. A more severe disease course was seen in the non-White ethnicity with both a shorter time to first relapse and a worse Expanded Disability Status Scale score at last follow-up. No patient treated with rituximab as first-line therapy relapsed. Optic neuritis at onset was associated with a poor visual outcome below 20/200, and a younger age at onset was associated with cognitive impairment.
International consensus on treatment protocols for children is required to reduce heterogeneity of treatment regimens used worldwide.
Paolilo RB, Hacohen Y, Yazbeck E, Armangue T, Bruijstens A, Lechner C, ApÓstolos-Pereira SL, Martynenko Y, Breu M, De Medeiros Rimkus C, Wassmer E, Baumann M, Papetti L, Capobianco M, Kornek B, Rostásy K, Da Paz JA, Ciccarelli O, Lim M, Saiz A, Neuteboom R, Marignier R, Hemingway C, Sato DK, Deiva K. Treatment and outcome of aquaporin-4 antibody-positive NMOSD: A multinational pediatric study. Neurol Neuroimmunol Neuroinflamm.
Retrospective, multicenter, and multinational study of patients with AQP4-Ab NMOSD aged <18 years at disease…
Developmental Medicine & Child Neurology - June 2020
The objective of this French paediatric cohort is to describe cognitive abilities through the evaluation of academic difficulties in children, aged 18 years and younger, with acute demyelinating syndromes (ADS) and myelin oligodendrocyte glycoprotein (MOG) antibodies.
Seventy‐six children (36 females, 40 males) were included in the study with a mean follow‐up of 4 years 7 months. Median age at disease onset was 9 years 1 month. 36 children relapsed and 20 had academic difficulties at the last follow‐up. Academic difficulties, as well as deep grey matter and putaminal lesions, were significantly more prevalent in children aged 10 years and younger.
We found that age at disease onset of 10 years and younger, acute disseminated encephalomyelitis at disease onset, and deep grey matter lesions were associated with academic difficulties.
Deiva K, Cobo-Calvo A, Maurey H, De Chalus A, Yazbeck E, Husson B, Vukusic S, Serguerra C, Horellou P, Marignier R, Kidbiosep Cohort. Risk factors for academic difficulties in children with myelin oligodendrocyte glycoprotein antibody‐associated acute demyelinating syndromes. Developmental Medicine & Child Neurology.
The objective of this French paediatric cohort is to describe cognitive abilities through the evaluation of academic difficulties in children, aged 18 years and younger, with acute…
Developmental Medicine & Child Neurology - February 2020
This study compared the scale of disability, fatigue, depression and quality of life of 26 children with MS (Multiple Sclerosis) and 11 children with ADS (Acute Demyelinating Syndromes).
Although not significant, severe fatigue was less frequently reported by patients with multiple sclerosis than children with ADS. Depression was reported more often in the multiple sclerosis group compared to the ADS group. Concerning the QoL in patients with multiple sclerosis, both parents and children reported poor emotional and school functioning. Physical and social functioning were rated as being good in both groups, and was significantly higher in the children’s group.
This clinical trial highlights the importance of fatigue and depression in children with ADS and particularly in paediatric onset multiple sclerosis. School difficulties and emotional functioning were the main concerns of parents and MS patients, and must be considered when requesting care and treatment proposal.
Florea A, Maurey H, Le Sauter M, Bellesme C, Sevin C, Deiva K. Service de Neurologie pédiatrique, CHU Bicêtre, APHP, Le Kremlin Bicêtre. Fatigue, Depression, and Quality of Life in Children With Multiple Sclerosis: A Comparative Study With Other Demyelinating Diseases. Developmental Medicine & Child Neurology.
This study compared the scale of disability, fatigue, depression and quality of life of 26 children with MS (Multiple Sclerosis) and 11 children with…
Journal of Neurology Neurosurgery & Psychiatry - January 2020
In PARADIGMS, a double-blind phase III trial in 215 paediatric patients with multiple sclerosis (MS) (10 to <18 years), fingolimod administered for up to 2 years significantly reduced the annualised relapse rate (ARR) and rate of new/newly enlarged T2 (n/neT2) lesions compared with interferon (IFN) β-1a.
In the treatment-naïve subpopulation, fingolimod reduced ARR and n/neT2 lesions by 85.8% and 53.4%, respectively versus INF β-1a, compared with 81.9% and 52.6% in the overall population. Model-based ARR reductions in younger patients (≤12 years) were 91.9%-94.6%. Twice as many IFN β-1a-treated than fingolimod-treated patients had worse EDSS scores at study end.
Fingolimod in paediatric MS was associated with consistent control of disease activity versus IFN β-1a and resulted in less disability progression for up to 2 years.
Deiva K, Huppke P, Banwell B, Chitnis T, Gärtner J, Krupp L, Waubant E, Stites T, Pearce GL, Merschhemke M. Consistent Control of Disease Activity With Fingolimod vs IFN β-1a in Pediatric-Onset Multiple Sclerosis. Journal of Neurology Neurosurgery & Psychiatry.
In PARADIGMS, a double-blind phase III trial in 215 paediatric patients with multiple sclerosis (MS) (10 to <18 years), fingolimod administered for up to…
New England Journal of Medicine – September 2018
PARADIGMS is the first, phase III, randomized, double-blind, double-placebo, clinical trial in childhood multiple sclerosis compared oral Fingolimod at a dose of 0.5 mg per day (0.25 mg per day for patients with a body weight of 40 kg) to intramuscular interferon beta-1a (IFNβ1a) at a dose 30 g per week.
Fingolimod significantly reduced the adjusted annualized relapse rate by 82%, compared to IFNβ1a treatment. The annualized rate of new or newly enlarged lesions on MRI was significantly lower with Fingolimod (4.39) than with IFNβ1a (9.27).
Adverse events, excluding relapses of multiple sclerosis, have been observed in 88.8% of patients treated with Fingolimod and 95.3% of those who received IFNβ1a. Serious adverse events have been reported in 16.8% of patients in the Fingolimod group, and 6.5% of patients in the IFNβ1a group.
Chitnis T, Arnold DL, Banwell B, Brück W, Ghezzi A, Giovannoni G, Greenberg B, Krupp L, Rostásy K, Tardieu M, Waubant E, Wolinsky JS, Bar-Or A, Stites T, Chen Y, Putzki N, Merschhemke M, Gärtner J PARADIGMS Study Group. Trial fo Fingolimod Versus Interferon beta-1a in Pediatric Multiple Sclerosis. New England Journal of Medicine.
PARADIGMS is the first, phase III, randomized, double-blind, double-placebo, clinical trial in childhood multiple sclerosis compared oral Fingolimod…











